The difference between validation and reproducibility
Validation and reproducibility are both important to guarantee high antibody quality but are two completely different concepts.
Validation
Before an antibody is introduced in our catalog, it has been thoroughly validated to ensure specificity. All validation data is presented on the product data sheet for each antibody.
At Atlas Antibodies, we have always worked extensively with antibody validation and with producing highly characterized antibodies our customers can trust.
Learn about antibody validation
Reproducibility
The reproducibility of a research antibody is the question of whether a new lot or new batch of an antibody performs equally compared to a previous lot or batch. Reproducibility should not be mixed up with validation or specificity of an antibody. At Atlas Antibodies, each new lot of the antibody is tested against its reference lot to make sure that new lots meet the same specificity criteria in each application. This is how we guarantee reproducibility.
Reproducibility in Immunohistochemistry
The standard test set-up for quality control of an antibody for the IHC application at Atlas Antibodies consists of a tissue microarray of more than 20 different samples of both normal and cancer tissues. The new lot of the antibody is tested and compared to the reference lot on consecutive sections from the tissue microarray.
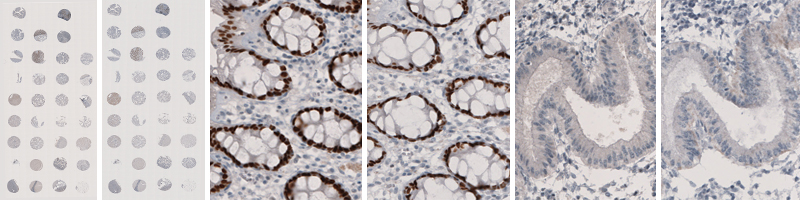
The images show examples from the quality control of antibody Anti-SATB2 (HPA001042) where a new lot is tested against its reference lot. The two left-hand images show the staining of 33 different tissues using lots B105190 and A43312 respectively. The two middle images show enlargements of the images of the staining of the rectum, both displaying nuclear staining in glandular cells. The two images to the right show the absence of staining in endometrium consistently in the two lots.

