9,000+ Western blot-validated antibodies
✔ We are the original manufacturer
✔ We validate on native protein from human tissues and cell lines
✔ We guarantee reproducible results with optimized protocols and support
✔ We believe in transparent data: WB images, protocols, and HPA links for every product
✔ We apply Enhanced Validation for over half our catalog
✔ Triple A Polyclonals™ and PrecisA Monoclonals™ data published by scientists worldwide
At Atlas Antibodies, we ensure every Triple A Polyclonal and PrecisA Monoclonal antibody is rigorously validated for Western blot applications to guarantee specific and reliable binding.
We confirm antibody binding by verifying that detected bands match the theoretical molecular weight of the target protein, while accounting for isoforms and post-translational modifications.
Our primary validation uses endogenous protein lysates from a broad panel of human tissues and cell lines. This approach closely reflects natural protein expression.
If suitable endogenous samples are unavailable, we employ recombinant full-length target proteins—commonly expressed in HEK-293 cells—to serve as positive controls.
Many antibodies are additionally validated on mouse and rat cell lysates, broadening their research applicability.
Detection is performed using peroxidase-labeled secondary antibodies combined with chemiluminescence and captured via advanced CCD camera systems, ensuring high-quality and reproducible WB imaging.
At Atlas Antibodies, we take great care to validate our antibodies and Enhanced Validation is performed as an additional layer of security in an application and context-specific manner.
To further confirm antibody specificity, we apply four enhanced validation methods:
The orthogonal validation method validates the antibody staining using a non-antibody-based method.
For example, the Western blot results are compared with RNA-Seq data for the same samples, using both positive and negative controls. Antibody specificity is confirmed when the antibody signal matches RNA levels in the evaluated samples.
For each antibody, two tissues or endogenous cell lines are chosen for validation: one with high RNA expression and the other with low or no RNA expression of the target protein. The cell lines are selected to express at least a five-fold difference between the RNA expression in the high and low samples.
In the enhanced validation data presented for the antibodies, the Western blot lanes in the high and low cell lines are displayed together with their corresponding RNA values.
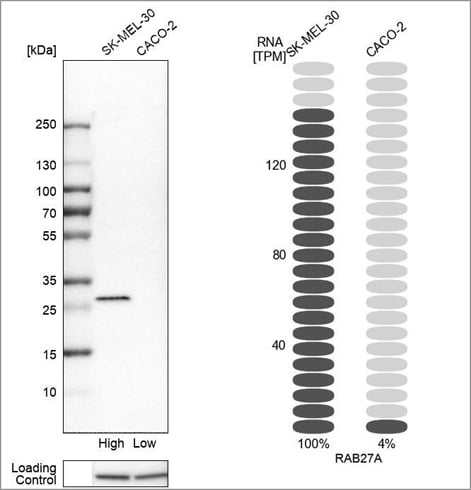
Example of orthogonal validation in WB, using the Anti-RAB27A antibody (HPA001333) in Western blot in the SK-MEL-30 and CACO-2 cell lines. On the right-hand side, bars representing the TPM values for RAB27A in the same cell lines are presented.
Genetic validation by siRNA knockdown is an enhanced method for validation where the target gene is downregulated. Antibody specificity is confirmed when the corresponding gene's knockdown levels correlate with a decrease in the antibody signal.
At Atlas Antibodies, two separate siRNA probes are employed to silence each target, and a loading control is added to ensure even loading and equal transfer over the gel. The knockdown is approved if at least 50% silencing is achieved for at least one of the two siRNA probes.
In the enhanced validation data presented for the antibody, the Western blot lanes in the control and knocked-down samples are displayed together with the loading control, and the relative remaining intensity after silencing is presented.
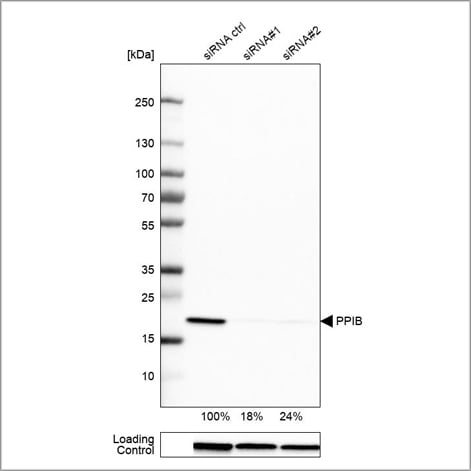
Example of genetic validation by siRNA knockdown in WB using the Anti-PPIB antibody (HPA012720). U-251 cells have been transfected with control siRNA and two target-specific siRNA probes. Downregulation of antibody signal confirms target specificity. The remaining intensity relative control lane is indicated as a percentage.
Validation by independent antibody is an enhanced method for validation where the antibody specificity is demonstrated by comparing at least two antibodies targeting the same protein with non-overlapping epitopes.
If the signals from the two antibodies correlate when compared across multiple samples, the antibodies validate each other.
In the validation data presented for the antibody, the Western blots from both antibodies are displayed together.
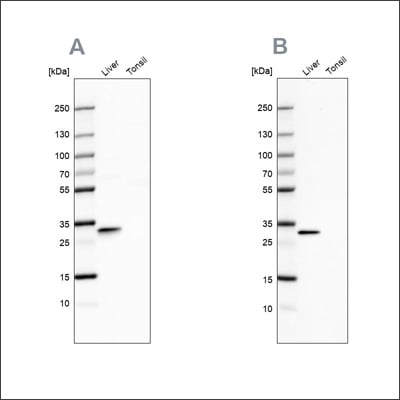
Example of independent antibody validation in WB using the Anti-PBLD antibody HPA038036 (A) shows a similar pattern to the independent antibody HPA038035 (B).
Recombinant expression validation is an enhanced method for validation where the antibody binding is confirmed using an over-expressed version of the target protein.
The method is applied to Western blot by comparing the antibody signal in a sample where the target protein has been recombinantly over-expressed with the signal from a control sample. Antibody specificity is confirmed when the antibody shows a strong band in the cell line with recombinant expression and no or faint band in the control line.
In the validation data presented for the antibody, the Western blot includes the over-expressed sample and the control sample in the same blot.
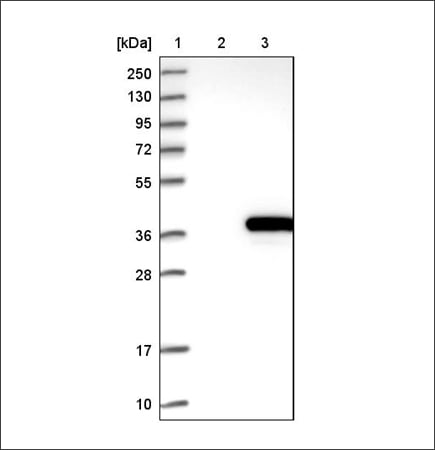
Example of recombinant expression validation in WB using the Anti-ACY3 antibody (HPA039219). Lane 1: marker, lane 2: negative control (vector only transfected HEK293T lysate), lane 3: ACY3 over-expression lysate (Co-expressed with a C-terminal myc-DDK tag (~3.1 kDa) in mammalian HEK293T cells, LY408962).
Use these recommended protocols for optimal results in Western blot using our antibodies. The protocols are optimized for Triple A Polyclonals and PrecisA Monoclonals.
Western blot Standard Protocol
Western blot Protocol - BSA Blocking