Immunohistochemistry (IHC)
Immunohistochemistry (IHC) is a crucial tool in histopathology for detecting proteins in tissues and cells. It uses antibodies to identify the presence and location of specific proteins in tissue samples, making it essential in both diagnosis and research.
Discover how our antibodies are validated for IHC and access the protocols needed for successful experiments.
In order to achieve the best results with our antibodies, make sure to use these recommended protocols. The protocols are optimized for our Triple A Polyclonals and PrecisA Monoclonals. Here you will also find our best advice for validation and troubleshooting in IHC.
IHC standard protocol
IHC protocol – Ventana Discovery XT
Our Triple A Polyclonals and PrecisA Monoclonals antibodies are validated through IHC staining of formalin-fixed, paraffin-embedded (FFPE) tissues in a tissue microarray (TMA) format.
IHC is a qualitative technique, so our skilled team performs thorough manual analysis to ensure antibody specificity across a wide range of human tissues.
For comprehensive protein expression profiling, the antibodies are tested on normal tissues from 144 individuals, representing 44 tissue types, and cancer tissues from 216 patients, including duplicate tumor samples from the 20 most common cancers.
A total of 576 tissue cores are immunostained and analyzed for each antibody. Cancer tissues include both high- and low-grade malignancies. Staining intensity, localization, and the proportion of stained cells are carefully evaluated and manually annotated for each tissue.
To further verify specificity, the validation performed for our antibodies is expanded with application-specific Enhanced Validation. In IHC, two different Enhanced Validation methods may be applied:
1. Orthogonal validation (verification done with a non-antibody-based method
2. Independent antibody validation (two antibodies targeting the same protein but binding to different parts of the protein thus verifying each other)
Currently, over 5,000 antibodies are validated using at least one of these two enhanced validation methods. Learn more about the methods below.
Watch the animation about Enhanced Validation
1. Orthogonal validation compares antibody signal to RNA
Orthogonal validation in IHC involves confirming antibody staining using a non-antibody-based method, like RNA-Seq. In IHC, this means comparing the antibody signal to RNA data from the same samples.
For validation, two tissues are selected for each antibody: one with high RNA expression and one with low or no expression of the target protein. The RNA levels in the two tissues should differ by at least five times.
The validation results show both the IHC staining for these two samples and their corresponding RNA data.
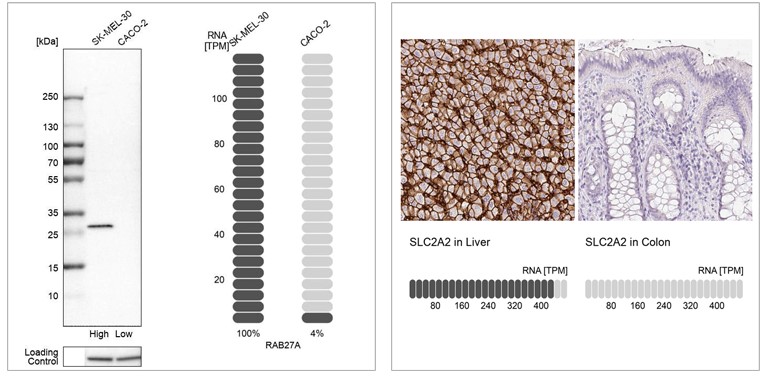
Example of orthogonal validation in IHC. IHC staining of liver and kidney tissues using the Anti-SLC2A2 polyclonal antibody (HPA028997). The corresponding RNA-Seq data (TPM values) for the same tissues are presentedat the bottom. Liver and colon samples were chosen because of their high and low SLC2A2 RNA expression, respectively.
2. Validation by independent antibody
This validation method involves confirming antibody specificity by comparing at least two antibodies that target the same protein but recognize different epitopes.
If the staining patterns from both antibodies are consistent across a range of relevant tissues, they validate each other. At Atlas Antibodies, 20-44 tissues are tested for each antibody, and four representative tissues are shown for each one.
The validation results include IHC staining images for both antibodies side by side.
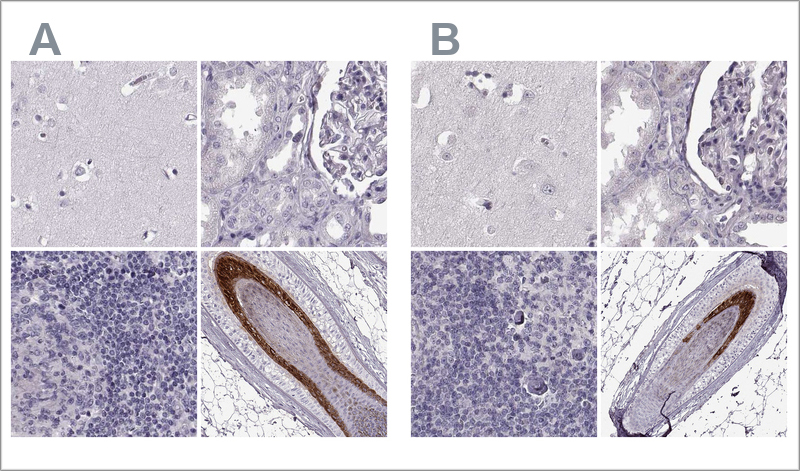
Example of validation by independent antibodies in IHC. The two Anti-TCHHL1 antibodies HPA063483 (left, A) and HPA042579 (right, B) target different regions of TCHHL1. The image shows IHC stainings of the two antibodies in the cerebral cortex, kidney, lymph node, and skin tissues. The antibody's staining pattern across positive and negative tissues is comparable, so the two antibodies validate each other.